
Science & Pipeline
We prioritize strong mechanism-of-action rationale, efficient pharmacology, and indication-aligned compound profiles. Programs are advanced with built-in inflection points for scientific and strategic decision-making.
How We Build
Swift™: Forward's Discovery Engine Unlocking Hard-to-Drug Targets
Forward’s Swift™ platform is a data-driven drug discovery engine designed to unlock high-potential, hard-to-drug targets. Our approach combines curated, drug-like libraries with elaborated screening assays and deep structural insights to enable rapid hit finding and lead optimization.
-
Agile Screens
Target tailored screens to identify high quality functional hits
-
Structural Insights
Cutting edge structural biology unlocks precise molecular designs
-
Intelligent designs
Data rich design cycles feeding our imagination and decisions
TNF signaling inhibitors
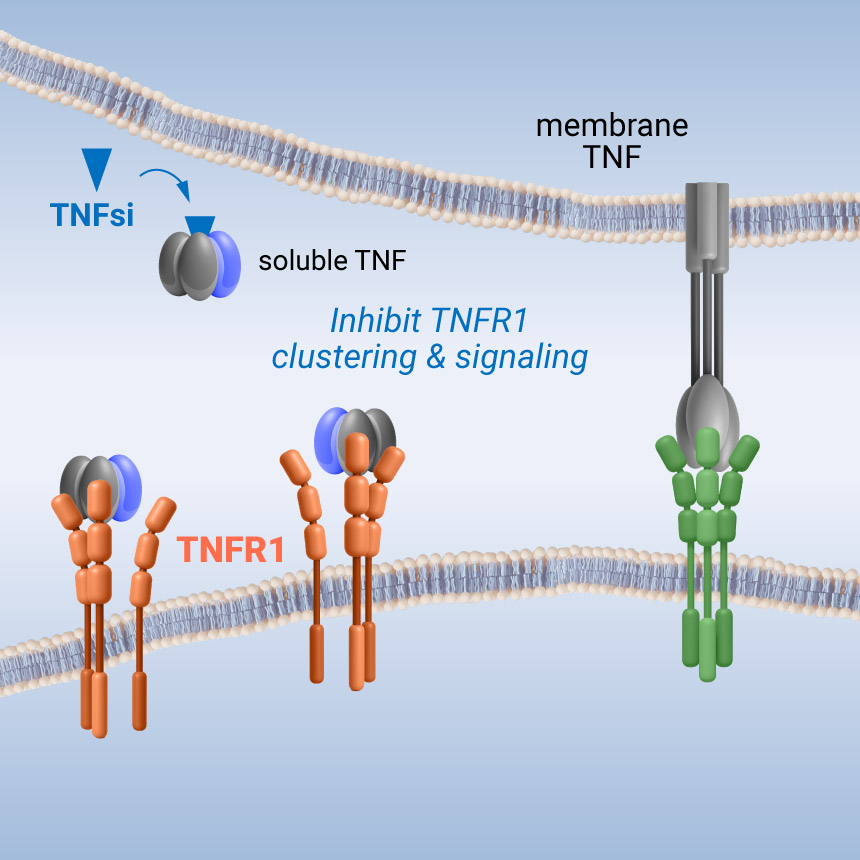
Our lead programs are designed to target soluble TNF – TNFR1 signaling, a central driver of inflammation and autoimmune diseases. Forward small molecules are designed to bind to a central pocket in soluble TNF, disrupting trimer symmetry, efficiently inhibiting trimeric TNFR1 signaling.
Unlike conventional biologics, the small molecule approach spares membrane TNF – TNF receptor signaling. Membrane TNF supports immune regulation, tissue repair and host defense. Our approach has the potential to provide targeted anti-inflammatory effects while minimizing unwanted immunosuppression – expanding potential uses and delivering a new treatment option for patients.
Soluble TNF Signaling inhibitor (TNFsi) Mechanism of Action and Differentiation
Selectively inhibit tnfr1 signaling
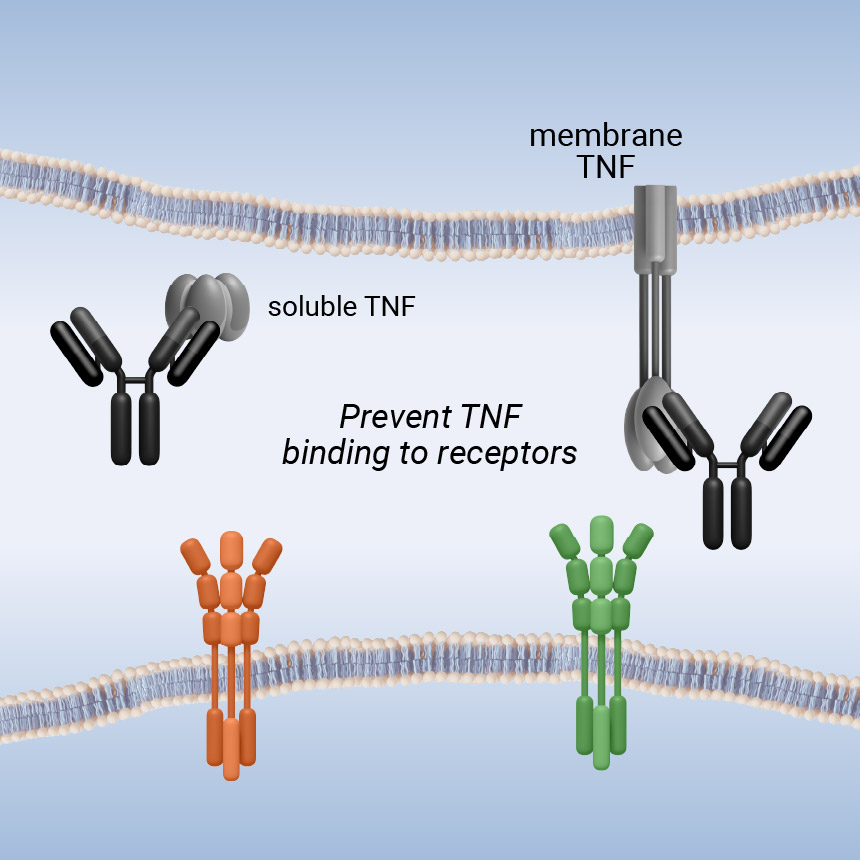
inhibit all TNF functions
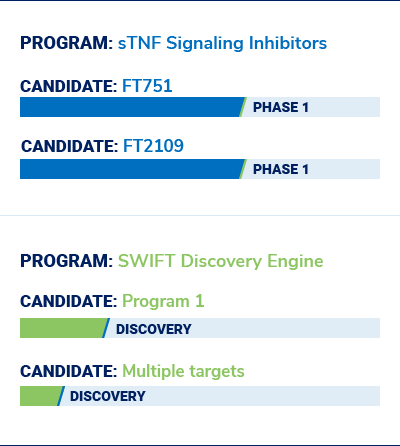
Our Pipeline
Expanded Access Policy
Forward Therapeutics is committed to developing innovative therapies for inflammation-driven diseases through rigorous clinical research. At this time, our investigational therapies are only available to patients through participation in clinical trials.
We do not currently offer an expanded access (compassionate use) program. This approach allows us to focus on generating the data necessary to fully evaluate the safety and efficacy of our investigational medicines and to seek regulatory approvals that can make these therapies broadly available to patients.
We encourage patients and healthcare providers interested in accessing our investigational therapies to explore ongoing clinical trials. Information about these studies can be found on public registries such as ClinicalTrials.gov and the EU Clinical Trials Register.
For additional information, please contact: info@forward-tx.com
